The global warming you have when you have no global warming
Like the sham whisky peddled in the 1970s and 80s, today’s fashionable narrative of global warming satisfies no one.
Climate realists and sceptics, knowing the facts, are frustrated by witless political surrender to counterfeit science, while climate agitators declare a crisis just to make something happen, since there’s no public belief, no political commitment and—fundamentally disturbing—no warming.
Yes, we have not been warming (well, nothing you could call a crisis …).
NZ & global warming hiatus lengthens
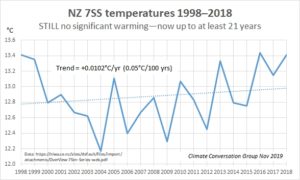
There’s been no New Zealand warming this century (right). The seven-station series (7SS) is the official national temperature data from NIWA. We published this graph in November 2017, after no net warming for 19 years. Data from 2017 and 2018 extend the hiatus to 21 years.
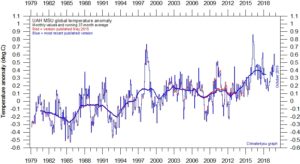
Globally, (UAH satellite data, left) there’s been a little warming from El Ninos in the last four years or so, but otherwise the warming stasis is persistent so far this century.
IPCC climate tale doesn’t add up
The sun pours more energy on us in an hour than mankind uses in a year, yet the Earth never overheats. Ignore the two decades of trivial warming before 2000—that ended 20 years ago. But, with all the energy soaking the Earth every day, a whopping 6000 Wh m-2, why does it never cause runaway warming? There’s only one possible answer: the existence of a powerful natural mechanism that continually discards energy to space and, adjusting to variations in solar strength, cloudiness, orbital irregularities and whatnot else, ensures that life survives.
The climate system presents occasional problems for we creatures, from floods to droughts to hurricanes, but a surfeit of energy isn’t among them, for it works constantly to dispose of the surplus. For over a billion years the climate system has kept temperatures in a range suitable for life, even while the solar energy poured into it was more than ample. The idea that mankind’s “extra” warming threatens the Earth is propaganda—it already handles extra warming, and any trifling heat from modern man-made CO2 gets mopped up along with the standard solar surplus.
The IPCC has never described this mighty cooling system and never produced evidence that mankind’s emissions of carbon dioxide cause dangerous (or even significant) warming. These complement each other, so millions accept that fake warming promises a perilous future. The exaggerated nightmares put out by extreme activists and news media patently embroider the IPCC reports without correction or reprimand from the IPCC or its scientists, to the point now that our children are seriously despondent about the imminent end of the world and suffering genuine mental illness.
In September, Peterri Taalas, the Finnish head of the WMO, spoke out against fearmongering, saying that, while climate scepticism had become less of an issue, the challenge was now coming from “doomsters and extremists”. The warmsters quickly denounced him, compelling an apology for his misleading comments.
Still, they ignore scepticism at their peril, since there are solid reasons to question the mechanisms behind the distressing IPCC narrative. Fundamental atmospheric processes and characteristics of the principal gases are not all as the IPCC reports describe them.
What happens to the vast energy shifted by water?
The principal greenhouse gas is water vapour (w.v.), which moves more energy around the atmosphere than all other greenhouse gases (GHG) combined. But what happens to it, where does it go?

Sumptuous, lovely to look at, but oodles of energy required to raise this sky eye candy. Lie back on the grass for five minutes and watch the billows churn with the heat released by the condensing vapour (click to enlarge). Here’s a video (1:43).
The energy involved in creating a cumulus cloud (like the one at right) is enormous. The fluffy cloud on a fine summer’s day, gleaming sumptuously, roughly cuboid, say a kilometre on a side, with a volume of a billion cubic metres, and a density of only half a gram per cubic metre, contains (believe it or not) about 500 tons of water—the weight of a large passenger jet loaded with 850 people, yet it literally floats on air. The largest of its water drops are (at first) microscopic.
The 500 tons require about 340 kWh of energy to evaporate, which is about a fortnight’s electricity usage for an average NZ couple and two cute, well-behaved children.
That cloud vaporised just minutes or hours before you see it, and it’ll all be back in the sea or soaking the land inside ten days. Actually, I’d like to know how scientists determine the atmospheric lifetime of water, since there’s no limit to the number of times a water molecule can change phase, so it might rise and fall many times before returning to the surface, and how could you track a single molecule through the atmosphere? Much of it could stay up there for years and why does it matter?
Cumulonimbus clouds are bigger and denser, up to 3.0 g/m3, can weigh 1,000,000 tons and require 2000 times more energy to evaporate: 680 MWh, or a month’s electricity for 2000 couples with two lovely Kiwi children.
Climate cooling a mighty double act of love?
Water’s first act of cooling occurs when it evaporates and soaks up about 685.47 watt-hours of energy per kilogram of water. Water’s second act of cooling occurs at altitude when it condenses and releases that same energy as sensible and latent heat back into the atmosphere. That doesn’t directly cool, but heats the air up. However, it’s at altitude, so most of the heat quickly rises and escapes to space. This goes on all day, every day. Along with oceanic and atmospheric transport to Arctic and Antarctic, the tremendous energy received each day in the tropics is efficiently distributed around the Earth and, some quickly, some slowly, jettisoned to space.
At any given moment, some 77 trillion tons of water are in the air as vapour and liquid. That water’s elevation keeps us alive and the energy to elevate it comes from the sun. Land and water heat up, warming the air, and the constant evaporation and condensation of those 77 trillion tons cools the air.
So we all live. Is this God, Gaia, mystery, mechanics or all five? Your thoughts are welcome.
Meandering mindlessly among minor matters
 Looking up Well-mixed Greenhouse Gases in Table 8.2 (p. 678) of the AR5, I was briefly captivated when I noticed a substance assigned the lowest radiative forcing (RF), of 0.0002 W m2. The gas is NF3, which Wikipedia tells us is nitrogen triflouride, “an extremely strong greenhouse gas” with a GWP 17,200 times greater than CO2. It perked my interest and I read on.
Looking up Well-mixed Greenhouse Gases in Table 8.2 (p. 678) of the AR5, I was briefly captivated when I noticed a substance assigned the lowest radiative forcing (RF), of 0.0002 W m2. The gas is NF3, which Wikipedia tells us is nitrogen triflouride, “an extremely strong greenhouse gas” with a GWP 17,200 times greater than CO2. It perked my interest and I read on.
NF3 is detectable in the atmosphere only at nanoscopic concentrations of 0.9 parts per trillion, with recent global emissions a mere 160 tons per year, things that should indicate a weak greenhouse gas.
The IPCC calculates the global warming potential (GWP) of many gases capable of absorbing energy from incoming solar radiation or outgoing long-wave IR. The few dozen CFCs and HCFCs total only 0.315 W m-2 but I think they list these nugatory quantities in desperation to buttress their abysmal case against CO2. Still, moving on…
CO2’s the control knob, eh?
 The IPCC narrative acknowledges that CO2 doesn’t cause much warming, but calls it the control knob, like turning the element up just a bit. When we emit CO2, they say, it absorbs heat from somewhere then re-radiates it, raising the temperature a little. The higher temperature evaporates more water, which strongly absorbs energy and causes even more warming (a positive feedback).
The IPCC narrative acknowledges that CO2 doesn’t cause much warming, but calls it the control knob, like turning the element up just a bit. When we emit CO2, they say, it absorbs heat from somewhere then re-radiates it, raising the temperature a little. The higher temperature evaporates more water, which strongly absorbs energy and causes even more warming (a positive feedback).
IPCC climate scientists describe this process of warming the atmosphere as “allowing [their word] more water vapour to evaporate”—about 7% more water for every Celsius degree of warming (tiny, since globally we’ve only seen one degree warming in a hundred years). They overlook the physical fact that water, on evaporating, extracts from its environment the energy to evaporate, amounting to 685.47 Wh/kg at 14 °C. So, yes, water might warm the air, but only after giving it a giant nudge of cooling!
I have searched for hours over the last few weeks but cannot locate the amount of radiative warming we might expect from w.v. I suspect that even increasing by 7% it’s nowhere near the enthalpy of vaporisation. But feel free to correct and thus inform me. Perhaps the IPCC might comment here to assist; I would be surprised but most grateful.
The IPCC fails to mention the GWP of water, with the highest heat capacity of any substance. They say it can’t be calculated, which means we can’t judge their theory, for how do we calculate the amount of warming from the positive feedback and therefore the amount of water evaporated? How do they calculate it? They must be guessing. Do they imagine we could agree that man-made warming was a problem without quantifying it?
Conclusions
Oh, the ecological agonies caused by that torment of timorous temperaments, carbon dioxide! The alarmist narrative nags us about our emissions and tells us the Earth is burning because of a mere 40%-odd increase since 1750, only 5% caused by us. Yet it’s still a trace gas, barely detectable, measured in parts per million, second in abundance to the trace gas argon, its meagre capacity to absorb energy among the tiniest of the puny. Meanwhile water vapour at 15,000 ppm and a veritable firepump of energy absorption goes galumphing about, soaking up tankerloads of energy officially ignored as “natural” though they overwhelm and neutralise our trivial contribution (as described above).
Near the surface of the Earth, evaporating water draws energy out of the environment from water, land and air, cooling it instantly. Shortly afterwards, in the cooler air at altitude, condensation jettisons all that heat, where it quickly rises even higher and dissipates to space. The water, now condensed, falls as rain, hail or snow, cooling the air and everything it falls on. Some of it evaporates as it falls through warmer air to continue transporting energy into the heavens.
So water automatically warms and cools the planet. An increase in warmth, however caused, evaporates more water, instantly cooling the surface and, a little later, setting that heat free high in the air, where it leaves the Earth forever. Occasional eruptions of volcanic aerosols shade and cool the Earth for a few months or years before dissipating. They can cause quite a wintry period but have always dispersed and the solar surplus quickly warms us up.
Clouds caused by more water vapour shade us and in a warming climate it’s reasonable to expect more clouds because there’s more water vapour going aloft, but we’re not sure. The IPCC guess that clouds are probably a positive (warming) feedback but admit they actually don’t know.
Water vapour in its various forms intercepts the sun’s largesse, dispersing, reflecting and elevating much of that energy, so while dispensing vital irrigation to plants, water vapour also protects this precious biosphere. All the while, our extra carbon dioxide increases plant life everywhere, making food for humans and animals alike more abundant and harming nothing.
[I admire the postscript regularly seen at WUWT advising readers to quote the actual words they comment on and I recommend it to you. – RT]
A few fundamental facts, reflections & basic calculations
Ignoring clouds, average global daily insolation is a whopping 6000 Wh m-2, and man-made forcing (2.29 W m-2) is just 0.038% of that. What is the global warming potential (GWP) of H2O? I cannot find it. There may be revelations in this page or elsewhere but sadly I’ve not yet seen it.
Discrepancies: IPCC reports the radiative forcing (RF) of natural CO2 = 1.82 W m-2, but RF of the man-made fraction (5%) of CO2 = 2.29 W m-2. The RF of WMGHG is 2.83 (2.54 to 3.12) W m–2.
Let’s look at the amount of water in the atmosphere. Kevin Trenberth’s 2005 paper The Mass of the Atmosphere: A Constraint on Global Analyses, estimates the mean mass of the whole atmosphere at 5.1480 × 1018 kg and of water vapour at 1.27 × 1016 kg.
Proportion of water: mass of water vapour ÷ mass of atmosphere ÷ 100
(1.27 × 1016 kg) ÷ (5.1480 × 1018 kg) × 100
= 0.0127 ÷ 5.1480 × 100
= 0.25% (2500 ppm)
This is a mass calculation based on ground-level pressure readings independently of processes occurring at different altitudes, and applies to the entire atmosphere. It does not reflect the actual proportion where the majority of water vapour is generated, at the surface, and must be somewhat low: half the atmospheric mass is below 5600 metres (18,000 ft), another 30% above it (to the tropopause), nearly all atmospheric water vapour and moisture is in the troposphere, and most of Earth’s weather takes place there. In other words, global averages don’t apply near the ground. Especially over the ocean, w.v. levels are well above 0.25%. This is just one area of complex climate interactions that need more study.
At different latitudes, w.v. concentration ranges from almost nothing to over 5% (50,000 ppm), and can climb to 30,000 ppm or 40,000 ppm under just increased relative humidity (no temperature increase, see Lenntech web calculator), and there’s a much higher proportion of w.v. in the lower half of the troposphere and over ocean (70% of the Earth) than near the tropopause. For these calculations I’ll assume 1.5% of w.v., which is 15,000 ppm, and the average global surface temperature, 14 °C.
Mass of water vapour at 1.5% mass of atmosphere:
(5.1480×1018 kg) × 0.015
= 77.22×1015 kg (77 trillion tons)
Convert mg/m3 to ppm
concentration (ppmm) = concentration H2O (mg m-3) x 24.45 ÷ molecular weight (18.01528)
assuming 25 °C and 1 atmosphere (1013.25 mbar)
For example: 15000 mg/m3 of H2O (18.01528 g/mol)
15000 x 24.45 ÷ 18.01528 = 20357.718 ppmm (2.04%)
The (latent) heat of vaporisation, called the enthalpy of vaporisation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a given quantity of that substance into a gas. The heat of vaporisation at 14 °C is 685.47 Wh/kg.
Kevin Trenberth (2005) states there are 77 trillion tons of water vapour and liquid in the atmosphere. We need to get a rough idea of the energy required to evaporate it.
Energy of evaporation at 14 °C: 77 trillion tons × heat of vaporisation
(77×1015 kg) × 685.47 Wh kg-1
= 52781.19×1015 Wh
= 52.78×1018 Wh
= 53×1012 MWh (trillion megawatt-hours)
Evaporating 77 trillion tons of water requires 53 trillion MWh. Can man-made CO2 provide that energy? It must do so in no more than 10 days, the lifetime of water vapour. Wikipedia tells us the surface area of Earth is 510.1×106 km²; IPCC (AR5, SPM, p. 13) says the man-made portion of CO2 is less than 5%, or 0.16 gigatons, and its radiative forcing is less than 2.29 W m-2.
Power of anthropogenic CO2 RF over 10 days (atmospheric lifetime of water vapour):
surface of Earth × RF of anthro CO2 × 240 hours
(510.1×1012m-2) × 2.29 Wm-2 × 240 h
= 280350.96 × 1012 Wh
= 280350.96 × 106 MWh
= 280 × 109 MWh
∴ in 10 days man-made CO2 exerts 280 billion (0.3 trillion) MWh on the earth, a tiny fraction (0.57) of the 53 trillion MWh required.
Mass of water evaporated by anthropogenic CO2:
power of anthropogenic CO2 RF for 10 days ÷ enthalpy of evaporation at 14 °C
(280 × 109 MWh) ÷ (685.47 Wh kg-1)
= (280 × 1015 Wh) ÷ (685.47 Wh kg-1)
= 0.4084788 × 1015 kg
= 409 billion tons
∴ man-made CO2 can evaporate only 0.4 trillion tons of water.
IPCC tells us natural CO2 RF is 1.82 W m-2 this is illogical as it’s smaller than the anthro fraction.
Power of natural CO2 RF for 10 days:
(510.1×1012 m²) × 1.82 W m-2 × 240 h
222811.68 × 1012 Wh
222.8 × 1015 Wh
222.8 × 109 MWh
∴ in 10 days natural CO2 exerts 222.8 billion MWh, only 80% of anthropogenic CO2.
Views: 260